Dr. William Kuo isn’t exactly a household name outside of the medical profession, but for patients who have been injured and their lives endangered by IVC (“inferior vena cava”) filters, Dr. Kuo represents their best hope for survival. Dr. Kuo is also speaking out about the dangers of this device, which has been implicated in over three dozen patient deaths and hundreds of serious internal injuries.
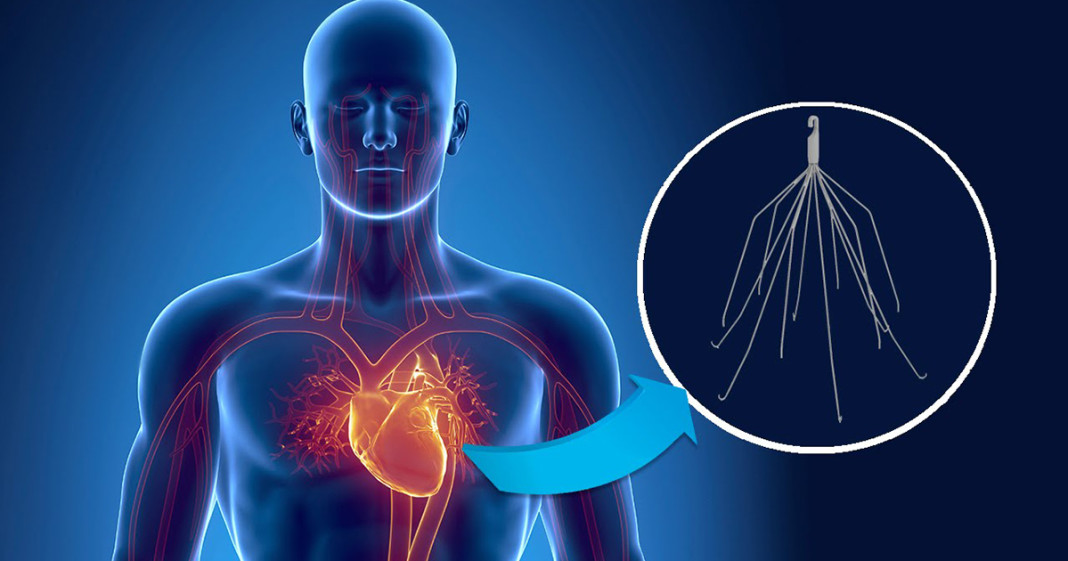
The IVC filter, a product of Bard Medical, is a small, spider-like, metal device designed to be implanted in a patient’s primary artery, the inferior vena cava. Its purpose is to trap blood clots that may travel up from the lower extremities and prevent them from entering the heart and the respiratory system. In most cases, the placement is meant to be temporary; they are primarily used for patients who have undergone joint surgery and may be in danger of blood clots that can cause a pulmonary embolism.
There have been two serious problems with removable IVC filters. In the first place, they are prone to fracturing and migration. Secondly, the devices are extremely difficult to remove – particularly once they have fallen out of place and/or broken apart.
This is what 69-year-old Christopher Svedise of San Francisco found out the hard way. He received a Bard G2 IVC filter in 2010 – the last year that Bard had the product on the market. This past October, Svedise was told by his doctor that his IVC filter had come loose and had migrated from its original site. It was now dangerously close to the heart itself. Because of this, two surgeons refused to perform the operation necessary to remove the device. That is when they called on Dr. Kuo.
William Kuo heads the IVC Filter Clinic at Stanford Health Care. He has developed a surgical method for removing damaged IVC filters and broken fragments. Even Dr. Kuo’s considerable surgical skills had limits, however: during Svedise’s surgical procedure, two fragments of his IVC filter broke off and floated into the heart muscle. Fortunately, Dr. Kuo and his surgical team were able to retrieve and remove them. He later said, “he’s very lucky.”
The major reason for these ongoing tragedies is an old story. It’s the corporate mentality of “profits over people.” In this case, C.R. Bard modified an existing product, already proven to be safe and effective, that was intended to remain in the patient’s body. The modifications were intended to make the filter removable – but it was far from being any sort of improvement. In an interview on Ring of Fire last November, Levin Papantonio litigation attorney Jeff Gaddy explained:
The manufacturers looked to get into the retrievable filter market and attempted to establish a niche…what they did was they took a permanent filter, which was a pretty decent product, and they weakened it.
Dr. Kuo has removed more than a thousand damaged removable IVC filters since 2006 – and most of them were manufactured by C.R. Bard, the company that proudly announces that it’s “Advancing the Delivery of Health Care.” It’s not the first time that Bard has marketed a dangerous product; last year, the company paid out $200 million to settle cases against it involving vaginal meshes that injured hundreds of women.
In September of 2015, an NBC News investigation revealed that executives at C.R. Bard were well aware of the dangers of their original Recovery IVC filter. Instead of issuing a recall, however, the company simply came out with a slightly modified version, which they called the “G2” (there is evidence that Bard executives committed criminal fraud in order to obtain FDA approval for the “new and improved” device). The later model was no better than the first, however. Despite hundreds of complaints to the FDA indicating that the G2 failed to correct the problems of the earlier model, Bard not only failed to correct the issues associated with the Recovery filter, but refused to recall the product – as would have been the moral and ethical thing to do.
In the great tradition of Corporate America, C.R. Bard can’t afford “morals” or “ethics” – even when human lives are at stake. According to Dr. Kuo:
All of the data that we’ve seen in our own studies, as well as other clinician researchers’, is that this device consistently fractures, consistently causes major complications. The number of complications, the frequency of severe failures makes it obvious that it was never safe to be implanted.
Dr. Kuo adds:
Whether it’s an ethical reason, a moral obligation, in the interest of public safety and patient safety, absolutely these devices should have been recalled…what we’ve learned the hard way is that we can no longer rely on medical device companies to do what’s in the best interest of the patient.
Nor can we rely on the FDA. That regulatory agency has been compromised by the for-profit health care industry. As Dr. Kuo points out, “We can no longer rely on the FDA to properly regulate these devices.”