NBC News may be a bit behind the curve, but at least the question has now been posed to the general public. Medical device manufacturer C.R. Bard continued to market and sell a product that was known to be defective, resulting in nearly 30 deaths and hundreds of serious injuries. Furthermore, there is strong evidence that Bard was willing to commit criminal fraud in order to keep the product on the market. The question now being asked is: why?
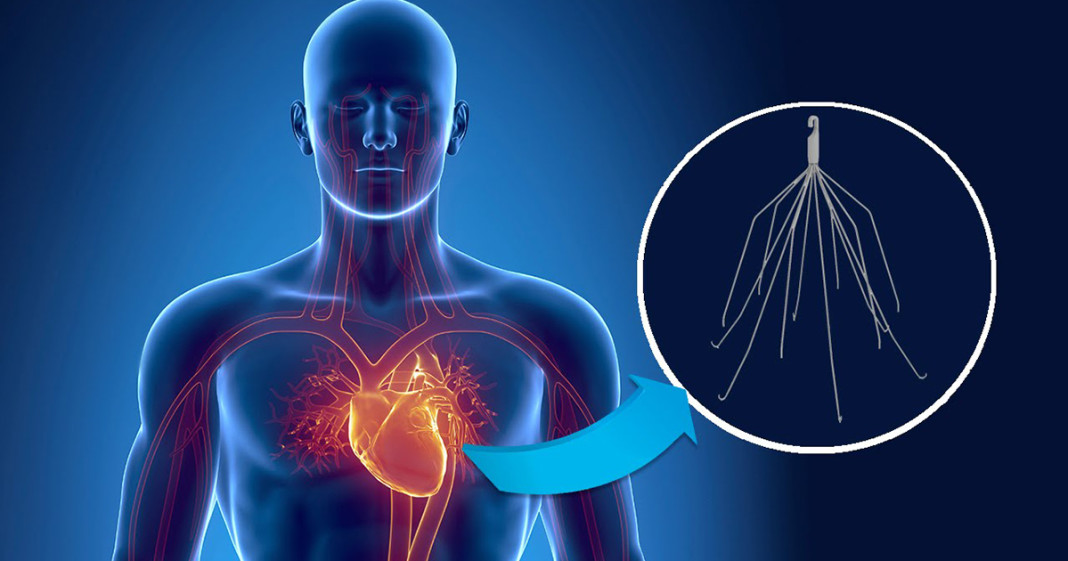
The product is known as the inferior vena cava, or IVC filter. This device, which bears a remarkable resemblance to the common cellar spider (better known as the “daddy longlegs”), is designed to be placed in the lower (inferior) artery leading into the heart, which carries blood from the lower body. The purpose is to trap blood clots resulting from joint surgery that may break loose and travel to the heart and lungs, preventing them from entering the cardiovascular system. What is known is that Bard’s IVC Filters have serious design flaws. Once implanted, the filters may pull loose, or fracture and disintegrate, releasing jagged bits of metal into the bloodstream – with serious, and even fatal results.
There were two models of the IVC filter involved. One was the G2, which was designed to replace an earlier model. Within four months of receiving FDA approval for the device in 2002, Bard began getting reports of adverse events involving fractures and migration. Despite these concerns, Bard chose to continue with the manufacture and sale of the G2, as well as the G2 Express – which was essentially the same device with a slight modification (for an explanation of how that got approved, go here).
Over a five-year period between 2005 and 2010, Bard sold over 160,000 units across the country.NBC News has now obtained a company memo, dated December 2005, which acknowledges that Bard’s Recovery filter had “an 11.5 times higher reporting rate for filter embolization deaths compared with all other vena cava filters.” That memo, which compares the Bard product with similar filters from other medical device manufacturers, states that “the Recovery has the least ability to resist migration” (in other words, breaking loose from its moorings and traveling into the heart muscle and cardiovascular system).
The story gets even more bizarre: near the end of summer 2015, a regulatory specialist who worked at Bard came forward with allegations that company executives had forged her signature of the FDA approval application. That employee, Kay Fuller, told investigative reporters that her own concerns over safety issues, as well as secrecy and lack of transparency over test results, led to her decision not to sign the application. Nonetheless, her signature – which Fuller states is not hers – appeared on the application.
Today, the problem has become so serious that one institution, Stanford Health Care, has established a clinic specializing in the removal of the filters. Dr. William Kuo, who heads the clinic, told NBC News, that “this device consistently fractures, consistently causes major complications.” So far, Kuo has performed more than 1,000 operations in order to remove IVC filters.
Bard’s willingness to ignore its own safety concern and even commit criminal fraud in order to profit from its product crosses a line that even the most egregious industry violators haven’t yet dared touch, according to Levin Papantonio attorney Brandon Bogle, who is heading up litigation against Bard. It’s not the first time Bard has been in trouble: recently, Bard paid out more than $200 million to settle lawsuits over its vaginal mesh products. Now, Bard has attracted the attention of the U.S. Senate Judiciary Committee. In September, committee chair Senator Charles Grassley (R-IA) sent a letter to the Acting Commissioner of the FDA, asking what the regulatory agency knew and what action has been taken. Senator Grassley reports that the Commissioner’s response was “incomplete.”
The sad fact is that the FDA has been compromised by corporate interests that are solely focused on profits – and are willing to inflict “collateral damage” in the form of patient injuries and deaths and pay out a few million dollars in judgments in order to protect and maximize those profits. Dr. Kuo summed it up to NBC News quite succinctly: “We can no longer rely on medical device companies to do what’s in the best interest of the patient. And we can no longer rely on the FDA to properly regulate these devices.”